Amateur Radiation Detection
and Experimentation Page
Purpose:
On this page, I discuss some of the experiments I've performed with the GM-10 and GM-45 geiger counter radiation detectors Click on the link for more information about these nuclear radiation detectors, and some of the interesting results I've found.Types of Nuclear Radiation:
There are three types of nuclear radiation which may be detected with a geiger counter:- Alpha Particles: Helium nuclei, generally emitted from heavy elements such as uranium and thorium. Alpha particles only travel a few inches in the air, and can be stopped by a piece of paper. Special geiger tubes with a mica window are necessary to detect them, as other windows will stop alpha particles.
- Beta Rays: Electrons moving at extremely high (often relativistic) speeds. They are more penatrating than alpha particles. They can pass through light elements, such as paper and aluminum (but only small thicknesses).
- Gamma Rays: Electromagnetic waves, similar to light, but at a much higher energy. Much more penetrating than alpha or beta radiations. High energy gamma rays can pass through several inches of metal. Note that X-Rays and Gamma Rays are really the same thing, the term X-Ray is used when the radiation is produced by electrons striking a material, such as in an X-Ray tube.
Available Now!
The low cost GM-10 Radiation Detector. 
Typical Geiger Counter Circuit:
GM Tube
10 M --------------
------------/\/\/\/-----*--------O |-----
| | -------------- |
+ | | |
----- | -----
500V --- | ---
| | _
| |--------) (----- Output
----- 100 pF
---
-
When a particle enters the tube, the gas is ionized, and can conduct current from the 500V DC supply. The 10M resistor limits the current to a safe level. A quenching gas (typically a halogen) stops the flow of current a few microseconds later. Thus, a pulse of current flows. This pulse is passed by the capacitor, which blocks the 500V DC. The output pulse can then go to an amplifier if necessary, and then some sort of counter. Typically, the counts are summed over a one minute period, and therefore the common unit is Counts Per Minute (CPM). The number of CPM depends on the size and efficiency of the GM Tube. A larger tube naturally will detect more particles. I've built a circuit which powers the GM tube strictly from the serial port handshake lines - no other power supply is necessary. The circuit then conditions the detected pulse and sends it back to the computer, where software can tally the number of pulses detected over a time period, typically CPM - Counts Per Minute.
This detector is now available as the GM-10, check out the details!
Sources of Radiation:
There are many Sources of Natural Radiation, radiation is all around us, naturally. Radon gas exists in most parts of the US, in varying levels depending upon where you live. Radon is produced from naturally occuring Uranium-238 in the soil. Radon is a problem in some areas today because homes are much more air-tight than they used to be. The radon gas enters the house through the basement. Thorium-232 also exists in the soil. Uranium and Thorium decay into numerous other radioactive isotopes before finally decaying into a stable element such as lead. And all this occurs naturally. In fact, the decay of uranium and thorium is the principle source of energy the heats the center of the Earth. Radiation existed long before Man, even though some would have you believe otherwise. The US Geological Survey has a Radon WWW Page. It contains a great deal of useful information, maps of radon levels in the US, and links to other sites.Note that even though most of the heavy elements are alpha emitters, they can still be dangerous, if they get inside your body. Your skin stops alpha particles if the source is outside your body, but your internal organs and tissues have no such protection. Radioactive dust can be hazardous. Radioactive dust? Read on...
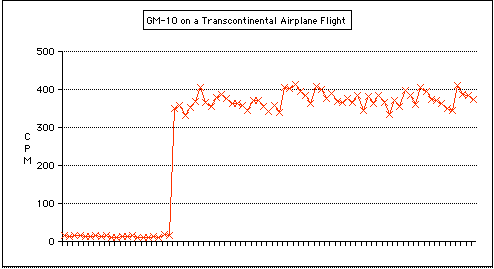
High Altitude Radiation from Airplane Flights
When you fly in an airplane, you're up above much of the Earth's atmosphere. It's the air that protects us from a lot of radiation due to cosmic rays coming from outer space. Recently I took a transcontinental flight, and of course a GM-10 Radiation Detector came along for the ride!The graph below shows the background radiation levels on the ground, as well as when we were at our crusing altitude (around 35,000 feet). Note the huge difference in radiation levels! The CPM (Counts Per Minute) went from about 12 on the ground to 360 in flight, or 30 times the level!
Radioactive Dust
Did you know that the dust that's in the air and settling all over your house (and computer monitor) is radioactive? It's true, it contains radioactive decay products from naturally occuring Uranium and Thorium. As an experiment, I wiped some dust from the TV screen onto a tissue, and placed it in front of the radiation detector. The reading went from a background reading around 10 CPM to around 1300 CPM, or 130 times the reading!This graph shows the radiation (in Counts Per Minute, CPM) over time, as the daughter products decay. In addition to plotting the raw data, I've also estimated the initial amounts of Radium B (Pb214), Radium C (Bi214) and Pb212:
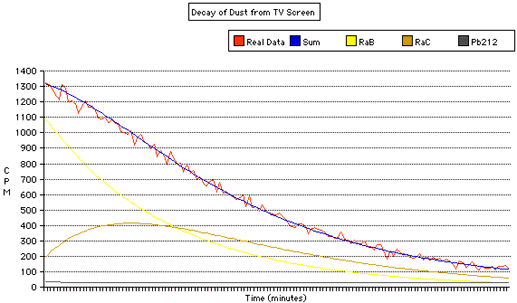
The Radon decays into Polonium, which them decays into Radium B, an isotope of Lead. This decays with a half life of about 27 minutes in Radium C, an isotope Bismuth. This decays with a half life of about 20 minutes into another Polonium isotope, which quickly (164 microseconds) decays into an isotope of Lead. The Lead decays with a very long half life of 22 years, so we don't get much radiation from it, or any further products.
The two Radium isotopes both undergo beta decay, and it is their radiation we detect in this experiment. Notice that we initially start with much more Radium B than Radium C. The Radium B decays, producing more Radium C, initially at a rate faster than the Radium C is decaying. So The amount of Radium C starts to increase. Eventually, there is not enough Radium B decaying into Radium C, and the amount of Radium C starts to drop.
Here's the process:
- Radon (Rn222) does an alpha decay into Polonium (Po218) with a half life of 3.824 days.
- Polonium (Po218) does an alpha decay into Lead (Pb214) with a half life of 3.05 minutes.
- Lead (Pb214) does a beta decay into Bismuth (Bi214) with a half life of 26.8 minutes.
- Bismuth (Bi214) does a beta decay into Polonium (Po214) with a half life of 19.8 minutes
- Polonium (Po214) does an alpha decay into Lead (Pb210) with a half life of 164 microseconds.
- Lead (Pb210) does a beta decay into Bismuth (Bi210) with a half life of 22.3 years.
- Bismuth (Bi210) does a beta decay into Polonium (Po210) with a half life of 5.01 days.
- Polonium (Po210) does an alpha decay into Lead (Pb206) with a half life of 138.38 days.
- Lead (Pb206) is stable.
I was doing laundry one day, and decided to check out the lint that collects in the lint filter in the clothes dryer. It produced counts around 240 CPM, again much higher than the background readings. This is probably due to the numerous uranium/thorium atoms and daughter products that are floating in the air, which get stuck to your clothes, and are sucked into the dryer.
Many parts of the US, including Maryland, where I live, have relatively high amounts of radon gas. This was never a problem in the past, but many modern homes are very air-tight, to reduce energy costs. This means that the radon gas is more likely to be trapped in the house, where it can decay into the much more dangerous daughter products.
Radon is much more of a problem for smokers. The radiation dust attaches itself to the smoke particles, and they get trapped to the sticky goo that gets stuck inside your lungs, bombarding your tissue with alpha radiation. (As if smoking was bad enough for you)
Radon Test Kits:
There are low cost ($15) test kits you can buy. You place a small cylinder (containing charcoal) in your basement. Radon daughter products deposit themselves in the container. You record the exact start and stop times for the collection period (typically a few days) and then mail the canister into a lab for analysis (lab fees built into the price). They use sensitive scintillator/PMT detectors to measure the amount of radiation, then adjust the results based on the collection period, and how long it took the canister to get to them in the mail. They then send you a report indicating the estimated radon level in the area, expressed as picocuries per liter of air. A curie is a measure of the activity of radiation. It is equal to 37 billion decays per second. The metric equivilent is the becquerel, which is one decay per second. So a picocurie is 0.037 decays per second. Currently the EPA considers 4 picocuries to be the safe limit. This would be 0.148 radon decays per second. Radon has a half life of about 3.83 days, this equates to around 100 thousand radon atoms per liter of air. Again, radon itself is not very dangerous, the risk comes from the radioactive daughter products (dust) that can get inside your body.Be sure to take a look at our new Radioactive Products and Other Sources Of Radiation page!
Other sources of information:
- Sources of Natural Radiaion
- www.raddetector.com - GM-10 and GM-45 radiation detectors (geiger counters)
- The Atomic Mac - A Periodic Table of the Elements for the Macintosh, also contains detailed nuclear information, half lives, decay modes, etc.
- Atomic Rocks - a great site for information on radioactive minerals.
- WHO WILL SPEAK FOR TRUTH? The Case of Nuclear Radiation
- LND, Inc. 3230 Lawson Boulevard, Oceanside, NY 11572 , (516) 678-6141, (516) 678-6704 - FAX. Mfg of Geiger tubes, Proportional Counters, Ionization Chambers, etc.
- Radioactivity in Nature - great info on naturally occuring radiation
- Terrestrial Gamma Radioactivity Map of the US showing levels of radiation due to Potassium, Thorium, and Uranium
- Uranium Minerals A great site showing where you can find your own radioactive minerals
- Natural Nuclear Reactor at Oklo, Gabon, West Africa
- Carbon 14 Dating
No comments:
Post a Comment